IDMP GUIDELINES FOR DIAGNOSIS AND MANAGEMENT OF INFLUENZA
DIAGNOSTICS
INFLUENZA DIAGNOSTICS REFERENCE TABLE
|
APEX Name |
Assay Type |
Viruses |
Sensitivity |
Specificity |
Sample |
COVID on same swab? |
Turn around time |
|
POCT FLU A and B RNA, QUAL RAPID1 |
Molecular |
Influenza A/B |
95%
|
>95% |
Nasal swab |
No |
15-20 min
Available in Parnassus and Mission Bay ED |
|
Influenza A/B/RSV PCR
|
Molecular |
Influenza A/B RSV |
>95% |
>95% |
NP/MNT +/- OP swab recommended (AN +/- OP accepted2) |
Yes |
4-8 h (STAT3)
12-24h (Routine) |
|
Respiratory Viral Panel PCR
|
Molecular |
Influenza A/B RSV Parainfluenza Metapneumovirus Rhinovirus Adenovirus |
>95% |
>95% |
NP/MNT +/- OP swab (AN +/- OP accepted2) or lower tract sample (BAL, ET aspirate) |
Yes |
4-8 h (STAT3)
24-72h (Routine) |
1Note: the POCT test is a rapid molecular test that is 95% sensitive (this is different than prior POCT antigen tests).
2Anterior nares (AN) swab is approximately 10-15% less sensitive than NP/MNT swab for respiratory virus testing
3STAT testing should be prioritized for new hospital admissions only when the results will change acute patient management
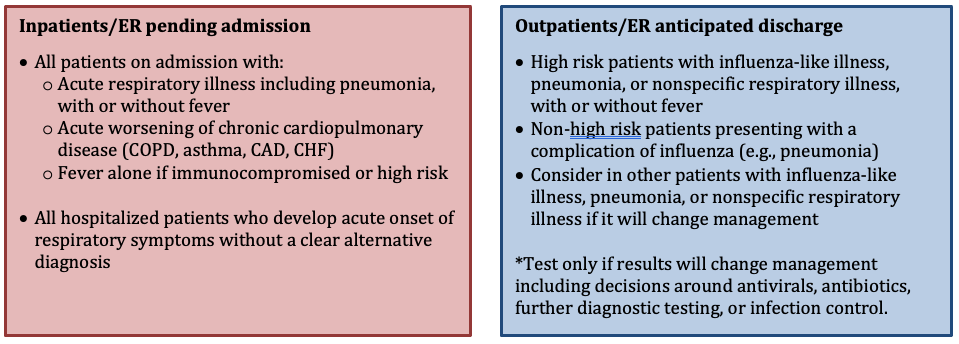
WHICH PATIENTS SHOULD BE TESTED FOR INFLUENZA DURING INFLUENZA SEASON?
Influenza Signs and Symptoms (usually abrupt onset)
- Respiratory symptoms: dyspnea, cough, chest pain
- Systemic signs and symptoms: chills, malaise, fatigue, myalgia with or without fever
- ENT symptoms: headache, sore throat, hoarseness (nasal congestion, rhinorrhea more common in children)
- GI symptoms: abdominal pain, vomiting (diarrhea more common in children)
Patients at High Risk of Complications
- Adults ≥ 65 years or children <5 years (especially <2 years)
- Chronic pulmonary, CV, renal, hepatic, heme, neuro/neurodevelopmental, metabolic disorders (incl. diabetes)
- Immunocompromised
- Pregnant or postpartum (within 2 weeks after delivery)
- Children <18 years receiving aspirin or salicylate containing medications (risk of Reye syndrome if get flu)
- American Indians/Alaska Natives
- Extreme obesity (BMI ≥40)
- Residents of chronic care facilities
WHICH TEST SHOULD I ORDER IN SYMPTOMATIC PATIENTS?
See the section above for guidance on indications for influenza testing. For the 2021-22 season, all patients who require influenza testing should also be tested for COVID.
Outside of Flu Season
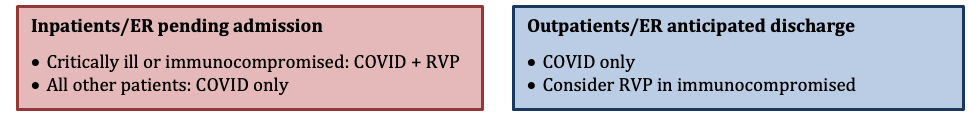
During Flu Season
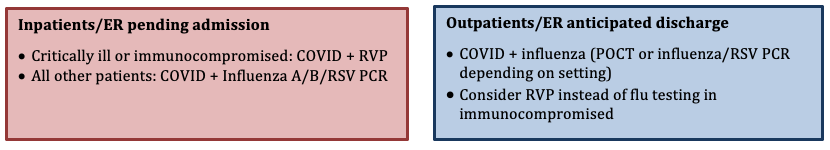
Important Notes on Testing
- The start of flu season will be indicated by UCSF Health with an institution-wide email based on internal influenza testing and SFDPH influenza surveillance. The onset of the influenza season varies but is usually in late December/early January in Northern California; the end of the season also varies.
- To maximize detection, respiratory specimens should be collected as close to illness onset as possible, preferably <4 days after symptom onset (but can and should be done later if patients do not present early
- In critically ill patients, send upper and lower respiratory tract samples for RVP to improve sensitivity for diagnosis of respiratory viral infection.
- Co-infection with influenza and SARS-CoV-2 is uncommon but can occur. A positive influenza test does not preclude SARS-CoV-2 infection, and vice versa.
TREATMENT
WHICH PATIENTS WITH INFLUENZA/SUSPECTED INFLUENZA SHOULD BE TREATED WITH ANTIVIRALS (DURING FLU SEASON)?
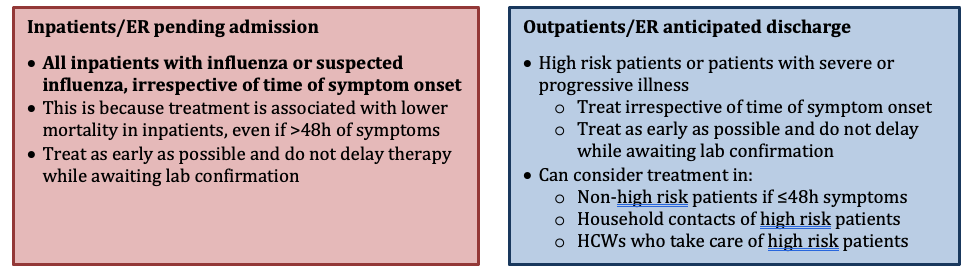
Important Notes on Indications for Treatment
- Household contacts of HCWs who take care of high risk patients should only be treated if they have a specific indication, not solely to prevent spread to the HCW.
- For young children, routine empiric influenza therapy in this age group is somewhat controversial. It is recommended to offer therapy to this group of patients, but individual treatment decisions may be considered via shared decision-making and incorporation of other clinical factors.
Drug Options
(Click on drug name for dosing guidance)
|
Drug |
Route |
Adverse Effects |
Comments |
|
PO |
Nausea/vomiting, rare neuropsychiatric effects. |
Drug of choice for most patients, including hospitalized patients, immunocompromised patients, and patients who are pregnant or breastfeeding. |
|
|
Zanamavir |
Inhaled |
Cannot use in intubated patients or those with respiratory disease (asthma/COPD) as it can cause cough, bronchospasm. |
Consider if patient cannot take PO although requires patient participation with use |
|
IV |
GI side effects, neutropenia |
Consider use in hospitalized patients with influenza in whom there is a concern for GI absorption that would limit the use of oral oseltamivir. |
|
|
PO |
Diarrhea |
Not routinely recommended given concerns about treatment emergent resistance. Can be considered for uncomplicated infection in immunocompetent outpatients. Not recommended for hospitalized patients, immunocompromised patients, or patients who are pregnant or breastfeeding given lack of data. |
Important Notes on Antiviral Therapy
- Please see IDMP (https://idmp.ucsf.edu) for dosing recommendations for oseltamivir in children and adults
- If you are considering zanamavir, peramivir, or baloxavir, please consult ID
- For ICU patients, treatment courses may be extended based on severity of illness and repeat RVP testing of lower respiratory tract samples. Please consult ID for assistance in these cases.
Considerations Regarding Bacterial Superinfection in Patients with Confirmed Influenza
- Bacterial superinfection is more common at clinical presentation in influenza than in COVID (~10% of hospitalized patients with influenza vs. <1-3% of hospitalized patients with COVID)
- If patients with influenza are started on antibiotics for CAP, consider early discontinuation (at 48-72h) if patient is clinically stable and there is a low suspicion for bacterial pneumonia based on labs and radiologic features.
PROPHYLAXIS
- These guidelines do not address the use of antivirals for chemoprophylaxis.
- For detailed guidance on indications for and use of antivirals for chemoprophylaxis, please see the CDC Antiviral Guidelines (section on chemoprophylaxis) which can be found here: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm
References
- Uyeki et al, IDSA 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza, CID 2019, 68:e1.
- CDC, Seasonal Influenza Antiviral Drugs, https://www.cdc.gov/flu/professionals/antivirals/index.htm, accessed September 27, 2021
- Metlay et al, Joint ATS/IDSA Guidelines for the Diagnosis and Treatment of Adults with CAP, AJRCCM 2019, 200:e45.