These dosing recommendations are meant as guidance based on available literature and should not replace clinical judgement. Antimicrobial dosing should take into account factors specific to the patient (weight, renal function), antimicrobial (pharmacokinetics, pharmacodynamics, toxicity) and disease-state.
Click here to submit a request to modify content on this page
84 Drugs
Acyclovir
|
Indication |
CrCl > 50 mL/min |
25 - 50 mL/min |
10 - 25 mL/min |
< 10 mL/min |
|---|---|---|---|---|
| Non-CNS HSV Infections | 5 mg/kg IV q8h | 5 mg/kg IV q12h | 5 mg/kg IV q24h | 2.5 mg/kg IV q24h |
| HSV encephalitis/ Disseminated VZV | 10 mg/kg IV q8h | 10 mg/kg IV q12h | 10 mg/kg IV q24h | 5 mg/kg IV q24h |
Dosing weights for acyclovir are controversial. The original package insert recommends dosing on ideal body weight in obesity. Subsequent studies suggest adjusted body should be used to reduce risk of underdosing. We recommend initial use of adjusted body weight in obesity with careful monitoring of renal function and mental status with potential for dose reduction as needed.
AmBisome (liposomal amphotericin B)
| Indication |
Dose |
Notes |
|---|---|---|
| Invasive fungal infections | 5 mg/kg IV q24h |
No adjustment for renal dysfunction; monitor serum creatinine and electrolytes |
| Mold prophylaxis (Heme/BMT) | 3 mg/kg IV q24h |
*Use Total Body Weight if Total Body Weight < Ideal Body Weight. If Total Body Weight >1.2 times Ideal Body Weight, use Adjusted Body Weight
IV Fluids: Give 500mL Normal Saline before and after AmBisome administration if able to tolerate
Restricted to ID or Antimicrobial Stewardship except:
1) Documented or suspected fungal pneumonia in a patient intolerant of or with contraindications to azoles
2) Prophylaxis against fungal infections in patients on the hematology/BMT service or lung transplant service
3) Empiric therapy for prolonged febrile neutropenia in hematology/oncology/BMT patient
Amikacin
| Indication | CrCl > 60 mL/min | 40 - 60 mL/min | 20 - 40 mL/min | < 20 mL/min |
|---|---|---|---|---|
| High-dose extended interval ("once-daily"): patients with normal renal function who are not morbidly obese or fluid overloaded | 15 mg/kg IV q24h |
Use traditional dosing or consult ID pharmacy for guidance |
||
| Traditional dosing: patients who do not qualify for high-dose extended interval dosing | 5-7.5 mg/kg IV q8h | 5-7.5 mg/kg IV q12h | 5-7.5 mg/kg IV q24h | 5-7.5 mg/kg IV x1 & consult ID pharmacy for maintenance dose |
Monitoring
|
Indication |
Monitoring |
|---|---|
| High-dose extended interval ("once-daily") |
Single level: Check random drug level 6-14 hours after the start of infusion. Compare to nomogram (below) Paired levels: Check peak drug level 2 hours after and random level 6-14 hours after infusion. Consult ID pharmacy for assistance. |
| Traditional dosing | Paired levels: Check peak drug level 30 minutes after end of infusion (goal 20-30 mg/L) and trough level immediately before next dose (goal <4 mg/L). |
Nomogram:
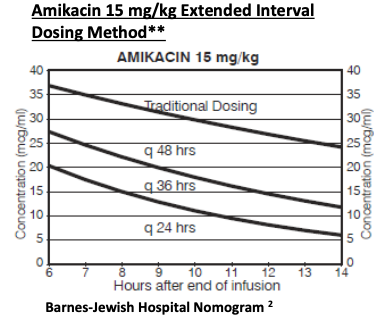
**If amikacin 20 mg/kg is used. Adjust the measured level with the following equation before plotting the level onto the Amikacin 15mg/kg Extended Interval Nomogram Level for the plot = Measured level x 0.75
Restricted to ID or Antimicrobial Stewardship for IV amikacin
Amoxicillin/clavulanate
| Indication | CrCl > 30 mL/min | 10 - 30 mL/min | < 10 mL/min |
|---|---|---|---|
| All Indications | 875/125 mg PO q12h | 500/125 mg PO q12h | 500/125 mg PO q24h |
Ampicillin
| Indication |
CrCl > 50 mL/min |
10 - 50 mL/min |
< 10 mL/min |
|---|---|---|---|
| Uncomplicated infection | 2 g IV q6h | 1 g IV q6h | 1 g IV q12h |
| Meningitis or endovascular infection | 2 g IV q4h | 2 g IV q6h | 1 g IV q8h |
Ampicillin/sulbactam (Unasyn)
| Indication | CrCl > 30 mL/min |
15 – 30 mL/min |
< 15 mL/min |
|---|---|---|---|
| All indications | 3 g IV q6h | 3 g IV q12h | 3 g IV q24h |
|
Carbapenem-resistant Acinetobacter baumannii |
3 g IV q4h | 3 g IV q8h |
3 g IV q12h
|
Extended Infusion Dosing (if recommended by ID or ID pharmacy)
| Indication | CrCl > 60 mL/min |
30-59 mL/min |
<30mL/min |
|---|---|---|---|
|
Carbapenem-resistant Acinetobacter baumannii |
9 gram IV q 8h (over 4 hours) |
6 grams IV q 8h (over 4 hours) |
Short infusion (follow the dosing recs of 3 g IV q 4h schematic) |
Artesunate
| Indication | Dose |
|---|---|
| All Indications | 2.4 mg/kg IV at 0 hours, 12 hours, and 24 hours, followed by 2.4 mg/kg IV q24h |
Restricted to ID or Antimicrobial Stewardship
Azithromycin
| Indication | Dose | Notes |
|---|---|---|
| Community-acquired pneumonia, ICU | 500 mg IV/PO q24h | No adjustment for renal dysfunction |
| Community-acquired pneumonia, non-ICU | 500 mg IV/PO x1 then 250 mg IV/PO q24h |
Aztreonam
| Indication |
CrCl > 50 mL/min |
10 - 50 mL/min | < 10 mL/min |
|---|---|---|---|
| All indications |
2 g IV q8h* |
2 g IV q12h | 1 g IV q12h |
*2 g IV q6h may be considered for meningitis in select patients based on clinical judgement (e.g. obesity, young age, augmented renal clearance, etc), or in select resistant gram-negative infections in combination with ceftazidime-avibactam (infuse both over 3 hours).
If used in combination with ceftazidime-avibactam and dosing intervals are the same, simultaneous administration is preferred.
Aztreonam lacks cross-reactivity with most other beta-lactams; however, most patients who have a recorded beta-lactam allergy can receive another beta-lactam through screening, test dosing, or skin testing. See Inpatient Allergy Guidelines
Baloxavir marboxil
| Indication | Dosage | Notes |
|---|---|---|
| Influenza treatment, uncomplicated, 40-79kg | 40 mg PO x1 | No renal dose adjustment |
| Influenza treatment, uncomplicated, >80kg | 80 mg PO x1 |
Restricted to ID or Antimicrobial Stewardship
Bezlotoxumab
| Indication | Dose | Notes |
|---|---|---|
| Secondary prevention from C. difficile infection | 10 mg/kg x 1 cumulative life time dose |
No renal adjustment In patients with a history of heart failure, bezlotoxumab use should be reserved for situations when the benefits outweigh risks. |
Restricted to ID or Antimicrobial Stewardship
Additonal criteria for inpatient administration: Must be expected to be hospitalized > 14 days after C. difficile episode
Cefazolin
| Indication | CrCl > 30 mL/min | 10 - 29 mL/min | < 10 mL/min |
|---|---|---|---|
| Uncomplicated Gram-Positive Infection | 1 g IV q8h | 1 g IV q12h | 1 g IV q24h |
| Gram-Negative or Complicated Gram-Positive Infection | 2 g IV q8h | 2 g IV q12h | 1 g IV q24h |
Cefepime
Short Infusion Dosing (PREFERRED)
| Indication | CrCl > 60 mL/min | 30 - 60 mL/min | 10 - 29 mL/min | < 10 mL/min |
|---|---|---|---|---|
| Non-severe infections including cystitis | 2 g IV q12h | 2 g IV q24h | 1 g IV q24h | 500 mg IV q24h |
| Severe infections including febrile neutropenia, meningitis, Pseudomonas aeruginosa | 2 g IV q8h | 2 g IV q12h | 2 g IV q24h | 1 g IV q24h |
Extended Infusion Dosing (if recommended by ID, ID pharmacy, or clinical pharmacy for select populations)
| Indication | CrCl > 60 mL/min | 30 - 60 mL/min | < 30 mL/min |
|---|---|---|---|
| Loading Dose* | 2 g x1 (over 30 minutes) | 2 g x1 (over 30 minutes) | Use short infusion |
| Maintenance: All Indications** | 2 g IV q8h (over 4 hours) | 2 g IV q12h (over 4 hours) | Use short infusion |
*Recommended to start maintenance dose 4 hours after loading dose
**Extended infusion data in meningitis are limited and may require further discussion with ID/ASP providers
Cefiderocol
| Indication | CrCl >120 mL/min | 60-119 mL/min | 30-59 mL/min | 15-29 mL/min | <15 mL/min |
|---|---|---|---|---|---|
| All Indications | 2 g IV q6h infused over 3 hours | 2 g IV q8h infused over 3 hours | 1.5 g IV q8h infused over 3 hours | 1 g IV q8h infused over 3 hours | 750 mg IV q12h infused over 3 hourse |
Restricted to ID or Antimicrobial Stewardship
Cefoxitin
| Indication | CrCl >50 mL/min | CrCl 30-50 mL/min | CrCl 10-29 mL/min | CrCl <10 mL/min |
|---|---|---|---|---|
| Standard dosing, NTM infection | 2 g IV q6h | 2 g IV q8h | 2 g IV q12h | 1 g IV q24h |
Ceftaroline
| Indication | CrCl > 50 mL/min | 30-50 mL/min | 15-30 mL/min | < 15 mL/min |
|---|---|---|---|---|
| Skin/Soft Tissue Infections or Community-acquired Pneumonia with low MRSA risk | 600 mg IV q12h | 400 mg IV q12h | 300 mg IV q12h | 200 mg IV q12h |
| Severe Infections, Pneumonia with documented or suspected MRSA | 600 mg IV q8h | 400 mg IV q8h | 300 mg IV q8h | 200 mg IV q8h |
Serious infections: ceftaroline dosed q12h may be an option in certain situations for CrCl < 50 mL/min and in iHD. Contact ID pharmacy for assistance.
Restricted to ID or Antimicrobial Stewardship
Ceftazidime
Short Infusion Dosing (PREFERRED)
| Indication | CrCl > 50 mL/min | 31 - 50 mL/min | 15 - 30 mL/min | < 15 mL/min |
|---|---|---|---|---|
| All Indications | 2 g IV q8h | 2 g IV q12h | 2 g IV q24h | 1 g IV q24h |
Extended Infusion Dosing (if recommended by ID, ID pharmacy, or clinical pharmacy for select populations)
| Indication | CrCl > 50 mL/min | 31 - 50 mL/min | < 31 mL/min |
|---|---|---|---|
| Loading Dose* | 2 g x1 (over 30 minutes) | 2 g x1 (over 30 minutes) | Use short infusion |
| Maintenance: All Indications | 2 g IV q8h (over 4 hours) | 2 g IV q12h (over 4 hours) | Use short infusion |
*Recommended to start maintenance dose 4 hours after loading dose
Ceftazidime/avibactam (Avycaz)
| Indication | CrCl > 50 mL/min | 31 - 50 mL/min | 16 - 30 mL/min | 6 - 15 mL/min | < 5 mL/min |
|---|---|---|---|---|---|
| All Indications | 2.5 g IV q8h | 1.25 g IV q8h | 0.94 g IV q12h | 0.94 g IV q24h | 0.94 g IV q48h |
Dosage recommendations are expressed as total grams of the ceftazidime/avibactam combination.
Restricted to ID or Antimicrobial Stewardship
Ceftolozane/tazobactam (Zerbaxa)
| Indication | CrCl >50 mL/min | 30-50 mL/min | 15-29 mL/min |
|---|---|---|---|
| Complicated urinary tract infection | 1.5 g IV q8h | 750 mg IV q8h | 375 mg IV q8h |
| Pneumonia, severe infections | 3 g IV q8h | 1.5 g IV q8h | 750 mg IV q8h |
Restricted to ID or Antimicrobial Stewardship
Ceftriaxone
| Indication | Dose | Notes |
|---|---|---|
| Standard Dose | 1 g IV q24h | No renal dose adjustment |
| Serious Infections, Non-enterococcal Endocarditis, Osteomyelitis, Septic Arthritis, Epidural Abscess, Intra-abdominal Infections, Liver Abscesses, Septic Shock | 2 g IV q24h | No renal dose adjustment |
|
Meningitis & Enteroccocal Endocarditis (in combination with ampicillin) |
2 g IV q12h | No renal dose adjustment |
Cefuroxime axetil
| Indication | CrCl >30 mL/min | 10-29 mL/min | <10 mL/min |
|---|---|---|---|
| All indications | 500 mg PO BID | 250 mg PO BID | 250 mg PO daily |
Cephalexin
| Indication | >30 mL/min | 15-29 mL/min | <15 mL/min |
|---|---|---|---|
| Most Indications | 500 mg PO QID or 1000 mg PO TID | 250-500 mg PO TID | 250-500 mg PO BID |
| Uncomplicated Cystitis or Streptococcal Pharyngitis | 500 mg PO BID | 250 mg PO BID | 250 mg PO daily |
Cidofovir
Adenovirus*
| High Dose Strategy | Criteria to initiate: CrCl >55 mL/min, SCr<1.5 mg/dL, urine protein <100mg/dL | Increase in SCr of 0.3-0.4 mg/dL | Increase in SCr of >0.5 mg/L or 3+ proteinuria |
|---|---|---|---|
| Induction (initial dosing) | 5 mg/kg IV once weekly WITH probenecid x 2 doses | 3 mg/kg IV once weekly WITH probenecid x 2 doses | Discontinue |
| Maintenance | 5 mg/kg IV once every 2 weeks WITH probenecid | 3 mg/kg IV every 2 weeks WITH probenecid | Discontinue |
*There may be alternative dosing available. Discuss with ID pharmacist to evaluate if patient meets criteria.
At UCSF, there is an adult inpatient order set available for use – IP Adult Cidofovir (VISTIDE) Order set
BK virus*
| High Dose Strategy | Criteria to initiate: CrCl >55 mL/min, SCr<1.5 mg/dL, urine protein <100mg/dL | Increase in SCr of 0.3-0.4 mg/dL | Increase in SCr of >0.5 mg/L or 3+ proteinuria |
|---|---|---|---|
| Induction (initial dosing) for BK viruria (hemorrhagic cystitis) in hematopoietic cell transplant recipients | 5 mg/kg IV once weekly WITH probenecid x 2 doses | 3 mg/kg IV once weekly WITH probenecid x 2 doses | Discontinue |
| Maintenance for BK viruria (hemorrhagic cystitis) in hematopoietic cell transplant recipients | 5 mg/kg IV once every 2 weeks WITH probenecid | 3 mg/kg IV every 2 weeks WITH probenecid | Discontinue |
| Low Dose Strategy | Criteria to initiate: CrCl >55 mL/min, SCr<1.5 mg/dL, urine protein <100mg/dL | Increase in SCr of 0.3-0.4 mg/dL | Increase in SCr of >0.5 mg/L or 3+ proteinuria |
|---|---|---|---|
|
BK viremia (BK nephropathy) in kidney transplant |
0.25 - 0.5 mg/kg IV once weekly WITHOUT probenecid | 0.25 - 0.5 mg/kg IV once weekly WITHOUT probenecid | Discontinue |
*There may be alternative dosing available. Discuss with ID pharmacist to evaluate if patient meets criteria.
At UCSF, there is an adult inpatient order set available for use – IP Adult Cidofovir (VISTIDE) Order set
Cytomegalovirus (CMV)
| Criteria to initiate: CrCl >55 mL/min, SCr<1.5 mg/dL, urine protein <100mg/dL | Increase in SCr of 0.3-0.4 mg/dL | Increase in SCr of >0.5 mg/L or 3+ proteinuria | |
|---|---|---|---|
| Induction (initial dosing) | 5 mg/kg IV once weekly WITH probenecid x 2 doses | 3 mg/kg IV once weekly WITH probenecid x 2 doses | Discontinue |
| Maintenance | 5 mg/kg IV once every 2 weeks WITH probenecid | 3 mg/kg IV every 2 weeks WITH probenecid | Discontinue |
At UCSF, there is an adult inpatient order set available for use – IP Adult Cidofovir (VISTIDE) Order set
IV Fluids: Give 1L NS over 30 minutes prior to cidofovir administration. If able to tolerate, give an additional 1L NS at the start or immediately after cidofovir administration.
Probenecid: 2g PO 3 hours prior to cidofovir, then 1g PO 2 and 8 hours after cidofovir (total 4g)
Restrict to ID or Antimicrobial Stewardship except:
1) For treatment or prophylaxis against viral infection in a BMT patient
Ciprofloxacin
| Indication | CrCl > 50 mL/min | 30 - 50 mL/min | < 30 mL/min |
|---|---|---|---|
|
400 mg IV q12h 500 mg PO BID |
400 mg IV q12h 500 mg PO BID |
400 mg IV q24h 500 mg PO daily |
|
| Pseudomonas infections, bloodstream infections, & induction therapy for Staphylococcus hardware infections +/- rifampin |
400 mg IV q8h 750 mg PO q12h |
400 mg IV q12h 500 mg PO q12h |
400 mg IV q24h 500 mg PO daily |
Clindamycin
| Indication | Dose | Notes |
|---|---|---|
|
600 mg IV q8h 450 mg PO q8h |
No renal dose adjustment | |
| Necrotizing Soft Tissue Infection & Group A Streptococcus Infection, Pelvic Inflammatory Disease | 900 mg IV Q8h | No renal dose adjustment |
Clofazimine
| Indication | Dosing | Notes |
|---|---|---|
| All Indications | 100 mg PO q24h | No adjustment for renal function |
Colistin IV
| Indication | Dosing |
|---|---|
| All IV Indications | 5 mg/kg IV x1 loading dose, then contact ID pharmacy for maintenance dose recommendations |
ZSFG: Non-formulary
Dosing for inhaled colistin per primary team protocols
Other more effective, less-toxic agents are available for most serious Gram-negative rod infections. In cases where an IV polymyxin is necessary, polymyxin B should be used preferentially for non-urinary tract infections in adults.
Restricted to ID or Antimicrobial Stewardship
*Inhaled use of colistin is allowed for unrestricted use in prophylaxis in lung transplant.
Dalbavancin
| Indication | CrCl > 30 mL/min | <30 mL/min |
|---|---|---|
| Single-dose regimen (skin/soft tissue infection) | 1500 mg IV x 1 dose | 1000 mg IV x 1 dose |
| Two-dose regimen (skin/soft tissue infection) | 1000 mg IV x 1 dose on day 1, then 500 mg IV X 1 on day 8 | 750 mg IV x 1 dose on day 1, then 375 mg IV X 1 on day 8 |
| Complicated Gram-positive infections (e.g. Native osteomyelitis)* | 1500 mg IV on days 1 & 8 | 1000 mg IV on days 1 & 8 |
|
*ID consult highly recommended for patient-specific recommendations. *For dosing in other complicated indications, contact ID or ID pharmacy. |
||
Other dosing strategies used for treatment of bone/joint infections or bacteremia.
Restricted to ID or Antimicrobial Stewardship
Daptomycin
| Indication | CrCl > 30 mL/min | < 30 mL/min |
|---|---|---|
| All indications | 8 – 10* mg/kg IV q24h | 8 – 10* mg/kg IV q48h |
*Doses up to 12 mg/kg may be indicated in treatment of some VRE infections - confer with ID/ID pharmacy.
*If Total BW >1.2 times ideal body weight, use adjusted body weight
Not effective in treatment of pneumonia.
Restricted to ID or Antimicrobial Stewardship
Doxycycline
| Indication | Dose | Notes |
|---|---|---|
| All indications | 100 mg IV/PO q12h | No renal dose adjustment |
Eravacycline
| Indication | Dosing | Notes |
|---|---|---|
| All Indications |
1mg/kg IV q12h Concomitant strong CYP3A4 Inducers: 1.5 mg/kg IV q12h |
No dose adjustment for renal dysfunction Severe hepatic impairment: 1 mg/kg IV q12h x2 doses, then 1 mg/kg IV q24h |
ZSFG: Non-formulary
Restricted to ID or Antimicrobial Stewardship
Ertapenem
| Indication | CrCl > 30 mL/min | < 30 mL/min |
|---|---|---|
| All indications | 1 g IV q24h | 500 mg IV q24h |
Ethambutol
This dosing aligns with current practices at the SFDPH TB Clinic (as of 5/2023) for treatment of tuberculosis. For patients in other counties, contact the respective TB clinics for dosing recommendations.
| Weight | CrCl > 30 mL/min | < 30 mL/min |
|---|---|---|
| 30-40 kg | 600 mg PO q24h | 20-25 mg/kg PO three times weekly |
| 41-50 kg | 800 mg PO q24h | 20-25 mg/kg PO three times weekly |
| 51-60 kg | 1000 mg PO q24h | 20-25 mg/kg PO three times weekly |
| 61-70 kg | 1200 mg PO q24h | 20-25 mg/kg PO three times weekly |
| 71-80 kg | 1400 mg PO q24h | 20-25 mg/kg PO three times weekly |
| 81-90 kg | 1600 mg PO q24h | 20-25 mg/kg PO three times weekly |
| > 91 kg | Consult ID or ID pharmacy | Consult ID or ID pharmacy |
Drug is available in 400mg and 100mg tablets.
This dosing aligns with current practices at the SFDPH TB Clinic (as of 5/2023) for treatment of tuberculosis. For patients in other counties, contact the respective TB clinics for dosing recommendations.
Fecal microbiota spores, live—brpk (Vowst)
| Indication | Dose | Notes |
|---|---|---|
| Secondary prevention from C. difficile infection |
Take 4 capsules taken orally once daily for 3 consecutive days - Refer to prescribing information for administration considerations |
No renal adjustment |
Restricted to ID/ASP for use in select adults with recurrent CDI who are expected to be hospitalized > 14 days after C. difficile episode.
Fidaxomicin
| Indication | Dosing | Notes |
|---|---|---|
| Clostridioides difficile infection | 200 mg PO q12h | No adjustment for renal dysfunction |
All use requires authorization from the Antimicrobial Stewardship Program or the consulting ID fellow
Fluconazole
| Indication | CrCl > 50 mL/min | CrCl 10 - 50 mL/min | CrCl < 10 mL/min |
|---|---|---|---|
| Oropharyngeal Infection | 100 mg IV/PO q24h | 50% of target dose IV/PO q24h | 25% of target dose IV/PO q24h |
| Esophageal Infection | 200 mg IV/PO q24h | 50% of target dose IV/PO q24h | 25% of target dose IV/PO q24h |
| Systemic/Severe Infections* |
≤ 80 kg: 400mg IV/PO q24h 81 – 100 kg: 600 mg IV/PO q24h > 100 kg: 800 mg IV/PO q24h |
50% of target dose IV/PO q24h | 25% of target dose IV/PO q24h |
*Higher doses may be necessary in some circumstances such as obese patients with severe infections
Flucytosine
| Indication | CrCl >40 mL/min | 20-40 mL/min | 10-20 mL/min | <10 mL/min |
|---|---|---|---|---|
| All Indications | 25 mg/kg PO q6h | 25 mg/kg PO q12h | 25 mg/kg PO q24h | 25 mg/kg PO q48h |
Foscarnet
Restricted to ID or Antimicrobial Stewardship except
1) For treatment or prophylaxis against viral infection in BMT patient
Fosfomycin
| Indication | CrCl > 50 mL/min | < 50 mL/min |
|---|---|---|
| Uncomplicated cystitis | 3 g PO x 1 dose | 3 g PO x 1 dose |
| Complicated cystitis | 3 g PO every 2 days x 3 doses | 3 g PO every 3 days x 3 doses |
Ganciclovir
| Indication | CrCl >70 mL/min | 50-69 mL/min | 25-49 mL/min | 10-24 mL/min |
|---|---|---|---|---|
| CMV Treatment | 5 mg/kg IV q12h | 2.5 mg/kg IV q12h | 2.5 mg/kg IV q24h | 1.25 mg/kg IV q24h |
| CMV Prophylaxis | 2.5 mg/kg IV q12h | 2.5 mg/kg IV q24h | 1.25 mg/kg IV q24h | 0.625 mg/kg IV q24h |
Gentamicin
Use traditional dosing or consult ID pharmacy for guidance
| Indication | CrCl > 60 mL/min | 40-60 mL/min | 20-40 mL/min | <20 mL/min |
|---|---|---|---|---|
| Gram-positive synergy | 1 mg/kg IV Q8h | Contact pharmacy for assistance | ||
| Gram-negative infections, high-dose extended interval ("once-daily"): patients with normal renal function who are not morbidly obese or fluid overloaded. | 5-7 mg/kg IV q24h | Use traditional dosing or contact pharmacy for assistance | ||
| Gram-negative infections, traditional dosing: patients who do not qualify for high-dose extended interval dosing | 1.6 mg/kg IV q8h | 1.5 mg/kg IV q12h | 1.5 mg/kg IV q12-24h | 2 mg/kg loading dose IV x1, contact pharmacy for maintenance |
*If Total BW > 1.2 times Ideal BW, use Adj BW.
Monitoring:
|
Indication |
Monitoring |
|---|---|
| Gram-positive synergy | Paired levels: Check peak drug level 30 minutes after end of infusion (goal 3-4 mg/L) and trough immediately before next dose (goal <1 mg/L) |
| Gram-negative high-dose extended interval ("once-daily") |
Single level: Check random drug level 6-14 hours after the start of infusion. Compare to nomogram below. Paired levels: Check peak drug level 1 hour after end of infusion and random level 6-14 hours after infusion. Consult ID pharmacy for assistance. |
| Gram-negative traditional dosing | Paired levels: Check peak drug level 30 minutes after end of infusion (goal 5 - 8 mg/L) and trough level immediately before next dose (goal <2 mg/L). |
Hartford Nomogram (7 mg/kg)

Urban-Craig Nomogram (5 mg/kg)
Imipenem/cilastatin
| Indication | CrCl > 60 mL/min | 30-60 mL/min | 15-30 mL/min | < 15 mL/min |
|---|---|---|---|---|
| Gram-negative or Nocardia infections* | 500 mg IV q6h | 250 mg IV q6h | 250 mg IV q8h | Use alternative of consult ID pharmacy |
|
Nontuberculous Mycobacteria** |
1000 mg IV q12h | 500 mg IV q12h | 250 mg IV q12h | Use alternative or consult ID pharmacy |
*There may be situations that Adult ID/ASP may recommend extended infusion in certain clinical scenarios
**Higher doses may be required for elevated imipenem MICs (8-16)
Restricted to ID or Antimicrobial Stewardship
Imipenem/cilastatin/relebactam
| Indication | CrCl >90 mL/min | 60-90 mL/min | 30-60 mL/min | 15-30 mL/min |
|---|---|---|---|---|
|
All Indications 1.25 g = imipenem 500 mg + cilastatin 500 mg + relebatam 250 mg |
1.25 g IV q6h | 1 g IV q6h | 750 mg IV q6h | 500 mg IV q6h |
Restricted to ID or Antimicrobial Stewardship
Isavuconazole
| Indication | Dose | Notes |
|---|---|---|
| All Indications | 372 mg IV/PO Q8h x 6 doses (total of 48h), then 372 mg Q24h | No renal dose adjustment |
372mg of isavuconazonium=200mg of isavuconazole
372mg of isavucazonium (prodrug) = 200mg of isavuconazole
Restricted to ID or Antimicrobial Stewardship except:
1) Documented or suspected fungal pneumonia in a patient with prolonged QTc interval
2) Prophylaxis against fungal infections in patients on the hematology/BMT service or lung transplant service with prolonged QTc interval or concurrent QTc prolonging medications.
Isoniazid
| Indication | Dosing | Notes |
|---|---|---|
| All Indications | 300 mg PO Q24h | No renal dose adjustment |
Any patients with confirmed or suspected active TB disease are required by law to be reported within 1 working day of identification to the TB Control Section. For more detail see: https://www.sfcdcp.org/tb-control/tuberculosis-information-for-medical-providers/reporting-tb-to-the-health-department/#:~:text=Reporting%20TB%20to%20the%20Health%20Department&text=Call%20(628)%20206-3398,554-3613%20for%20urgent%20reporting
Letermovir
| Indication | CrCl >10 mL/min | <10 mL/min |
|---|---|---|
| All Indications |
480 mg IV/PO q24h With concomitant cyclosoprine: 240 mg IV/PO q24h |
No dosing recommendations available |
ZSFG: Non-formulary
Restricted to ID or Antimicrobial Stewardship except for:
1) Adult malignant hematology service - for prophylaxis only per service guidelines
2) Prophylaxis of CMV disease in high-risk kidney transplant recipients
3) Continuation of current outpatient therapy
Levofloxacin
| Indication | CrCl > 50 mL/min | 20-49 mL/min | <20mL/min |
|---|---|---|---|
| Urinary tract infections | 500mg IV/PO Q24h | 500 mgx1, then 250mg IV/PO Q24h | 500mg x1, then 250mg IV/PO Q48h |
| Other indications | 750mg IV/PO Q24h | 750mg IV/PO Q48h | 750mg x1, then 500mg IV/PO Q48h |
Avoid co-administration of the oral formulation with divalent/trivalent cations (e.g. calcium, magnesium, zinc) - separate by at least 2 hours.
Linezolid
| Indication | Dose | Notes |
|---|---|---|
| All Indications | 600 mg IV/PO q12h | No renal dose adjustment* |
| Mycobacterial Infections | 600 mg IV/PO q24h | No renal dose adjustment |
*In clinically stable patients with CrCl <30 mL/minute and an anticipated treatment course >10 days, some experts suggest reducing dose to 300 mg IV/PO twice daily after 72 hours to reduce the risk of thrombocytopenia. Therapeutic drug monitoring may be utilized. Contact ID or ID pharmacy for assistance.
All use in pediatric patients at UCSF requires approval from the Pediatric Antimicrobial Stewardship Program or pediatric ID physician
All use at ZSFG requires approval by ID pharmacist or ID fellow.
Maribavir
| Indication | ||
|---|---|---|
| All Indications | 400 mg PO BID | No adjustment for renal dysfunction |
Dose increase required when administered with drug metabolizing enzyne inducers.
Restricted to ID or Antimicrobial Stewardship except:
1) Continuation of prior therapy
Meropenem
Short Infusion Dosing (PREFERRED)
| Indication | > 50mL/min | 26 - 50 mL/min | 10 - 25 mL/min | < 10mL/min |
|---|---|---|---|---|
| Standard Dosing | 1 g IV q8h | 1 g IV q12h | 500 mg IV q12h | 500 mg IV q24h |
| Meningitis, Cystic Fibrosis | 2 g IV q8h | 2 g IV q12h | 1 g IV Q12h | 1 g IV q24h |
Extended Infusion Dosing (if recommended by ID, ID pharmacy, or clinical pharmacy for select populations)
| Indication | > 50mL/min | 26 - 50 mL/min | < 26 mL/min |
|---|---|---|---|
| Loading Dose* | 1 g x1 (over 30 minutes) | 1 g x1 (over 30 minutes) | Use short infusion |
| Maintenance: Standard Dosing | 1 g IV q8h (over 3 hours) | 1 g IV q12h (over 3 hours) | Use short infusion |
| Maintenance: Meningitis**, Cystic Fibrosis | 2 g IV q8h (over 3 hours) | 2 g IV q12h (over 3 hours) | Use short infusion |
*Recommended to start maintenance dose 4 hours after loading dose
**Extended infusion data in meningitis are limited and may require further discussion with ID/ASP providers
Meropenem/vaborbactam
| Indication | eGFR >50mL/min/1.73m2 | 30-50 mL/min/1.73m2 | 15-30 mL/min/1.73m2 | <15 mL/min/1.73m2 |
|---|---|---|---|---|
|
All Indications 4 g = meropenem 2 g + vaborbactam 2 g |
4 g IV q8h over 3 hours | 2 g IV q8h over 3 hours | 2 g IV q12h over 3 hours | 1 g IV q12h over 3 hours |
Restricted to ID or Antimicrobial Stewardship except:
1) Continuation of prior therapy
Metronidazole
| Indication | CrCl >10 mL/min | CrCl < 10 mL/min |
|---|---|---|
| Standard Dose | 500 mg IV/PO Q8h | 500 mg IV/PO Q12h |
| Bacterial vaginosis | 500 mg IV/PO Q12h | 500 mg IV/PO Q12h |
|
Intra-abdominal infections (excluding C. difficile) |
500 mg IV/PO Q12h | 500 mg IV/PO Q12h |
| Vaginal Trichomoniasis | 500 mg IV/PO Q12h | 500 mg IV/PO Q12h |
Micafungin
| Indication | Dose | Notes |
|---|---|---|
| Esophageal candidiasis | 150 mg IV daily |
Dosage adjustment not required in renal or hepatic dysfunction |
| Prophylaxis against Candida in patients with HSCT, neutropenia, hematologic malignancy, or solid organ transplant | 50-100 mg IV daily | |
|
Candidemia Invasive candidiasis Empiric treatment, febrile neutropenia Empiric treatment, non-neutropenic ICU patients |
100 mg IV daily |
Restricted to ID or Antimicrobial Stewardship except:
1) Documented sterile site (not urine or respiratory) infection with microbiologically confirmed Candida glabrata or Candida kruseii
2) Documented sterile site infection (not urine or respiratory) infection with yeast, pending species identification
3) Prophylaxis against fungal infections in patients on the hematology/oncology/BMT service with intolerance of or contraindications to azoles
4) Empiric therapy for prolonged febrile neutropenia in hematology/oncology/BMT patient
Miltefosine
| Indication | Dose | Notes |
|---|---|---|
| Amebic meningoencephalitis |
< 45 kg: 50 mg PO twice daily with food >= 45 kg: 50 mg PO three times daily with food |
No renal adjustment |
| Leishmaniasis |
30-44 kg: 50 mg PO twice daily with food >= 45 kg: 50 mg PO three times daily with food |
No renal adjustment |
Restricted to ID or Antimicrobial Stewardship with exceptions:
1) Continuation of therapy
Minocycline
| Indication | Dosing | Notes |
|---|---|---|
| All Indications | 200 mg IV/PO x1, then 100 mg IV/PO q12h | No adjustment for renal dysfunction |
*There may be certain situations that may require higher dosing (200 mg IV/PO q12h). Speak to ASP/ID pharmacy if needed.
Molnupiravir
| Indication | CrCl >50 mL/min | CrCl <50 mL/min |
|---|---|---|
| All Indications | 800 mg PO q12h | No renal dose adjustment |
Moxifloxacin
| Indication | Dosing | Notes |
|---|---|---|
| All Indications | 400 mg IV/PO q24h | No adjustment for renal dysfunction |
Nafcillin
| Indication | Dosing | Notes |
|---|---|---|
| Meningitis, osteomyelitis, bloodstream infection, or endovascular infection | 2 g IV Q4h |
No renal dose adjustment |
Nirmatrelvir/ritonavir (Paxlovid)
| Indication | CrCl >60 mL/min | 30-60 mL/min | <30 mL/min |
|---|---|---|---|
| All Indications | 300/100 mg PO BID | 150/100 mg PO BID | Not recommended |
Review medications for potential drug interactions.
Nitrofurantoin (Macrobid)
| Indication | CrCl > 60 mL/min | < 60 mL/min |
|---|---|---|
| Cystitis Treatment | 100 mg PO q12h | Data limited for CrCl<60 mL/min; consider alternatives |
| Cystitis Prophylaxis | 100 mg PO q24h |
Omadacycline
| Indication | Dosing | Notes |
|---|---|---|
|
All Indications PO: must fast for at least 4 hours before and 2 hours after dose |
200 mg IV x1, then 100 mg IV q24h 450 mg PO daily x2 doses, then 300 mg PO daily |
No adjustment for renal dysfunction |
Loading doses may not be required when used for non-tuberculous mycobacterial infections
Oral formulation
Restricted to ID or Antimicrobial Stewardship except:
1) Continuation of prior therapy
Oritavancin
| Indication | Dosing | Notes |
|---|---|---|
| All Indications | 1200 mg IV x1 over 3 hours | No adjustment for renal dysfunction |
Restricted to ID or Antimicrobial Stewardship
Oseltamivir
| Indication | CrCl > 60 mL/min | 31-60mL/min | 10-30mL/min |
|---|---|---|---|
| Influenza treatment | 75 mg PO BID | 30 mg PO BID | 30 mg PO daily |
| Influenza prophylaxis | 75mg PO daily | 30mg PO daily | 30mg PO every other day |
Penicillin G
| Indication | CrCl > 50 mL/min | CrCl 10 – 50 mL/min | CrCl < 10 mL/min |
|---|---|---|---|
| Neurosyphilis, meningitis | 4 million units IV q4h | 3 million units IV q4h | 3 million units IV q6h |
| Endovascular, bacteremia | 3 million units IV q4h | 3 million units IV q6h | 2 million units IV q6h |
Pentamidine IV
| Indication | CrCl > = 10 ml/min | CrCl < 10 ml/min |
|---|---|---|
| Pneumocystis pneumonia | 4 mg/kg IV q24h | 4 mg/kg IV q36h |
*The current prescribing information does not provide specific renal adjustments and may need to consider other dosing outside of these recommendations.
*The current prescribing information does not provide specific renal adjustments and may need to consider other dosing outside of these recommendations.
Restricted to ID or Antimicrobial Stewardship
Peramivir
| Indication | CrCl >50 mL/min | 30-50 mL/min | 10-30 mL/min | <10 mL/min |
|---|---|---|---|---|
| All Indications | 600 mg IV q24h | 200 mg IV q24h | 100 mg IV q24h | 100 mg IV x1, then 15 mg IV q24h |
Restricted to ID or Antimicrobial Stewardship
Piperacillin/tazobactam (Zosyn) EXTENDED INFUSION
| Indication | CrCl > 20 mL/min | <20 mL/min |
|---|---|---|
|
UCSF: All Infections, including documented/suspected Pseudomonas Exclusion criteria for EXTENDED INFUSION: resistant or intermediate susceptibility organism, cystic fibrosis, peri-procedural areas, insufficient IV access ZSFG: Use EXTENDED INFUSION when recommended by ID pharmacist or ID fellow |
Loading dose = 4.5 g IV over 30 min x1, then 4.5 g IV infused over 4h every 8h (starting 4h after loading dose) | Use SHORT INFUSION piperacillin/tazobactam |
See Adult Extended Infusion Piperacillin/Tazobactam Protocol for additional details.
UCSF: PREFERRED dosing strategy if no exclusions
ZSFG: Available upon ID pharmacy or ID fellow recommendation
Piperacillin/tazobactam SHORT Infusion (SI) (Zosyn)
| Indication | > 50 mL/min | 10 – 50 mL/min | < 10 mL/min |
|---|---|---|---|
|
UCSF: Use SHORT INFUSION only for patients excluded from EXTENDED INFUSION dosing ZSFG: Use SHORT INFUSION unless EXTENDED infusion is recommended by ID |
|||
|
Non-Pseudomonas infections |
3.375 g IV q6h |
3.375 g IV q8h |
2.25 g IV q8h |
| Documented/Suspected Pseudomonas aeruginosa infection |
CrCl > 20 mL/min: 4.5 g IV q6h |
CrCl < 20 mL/min: 3.375 g IV q8h |
|
ONLY for patients excluded from EI dosing at UCSF. Exclusion criteria for EI: Resistant or intermediate organism, cystic fibrosis, periprocedural areas, insufficient IV access
Plazomicin
| Indication | CrCl >60 mL/min | 30-60 mL/min | 15-30 mL/min | <15 mL/min |
|---|---|---|---|---|
| All Indications | 15 mg/kg IV q24h | 10 mg/kg IV q24h | 10 mg/kg IV q48h | Not studied |
If Total BW >1.2 times Ideal BW, use Adjusted BW
Restricted to ID or Antimicrobial Stewardship
Polymyxin B
| Indication | Dosing | Note |
|---|---|---|
| All Indications | 2.5 mg/kg IV x1, then 1.5 mg/kg IV q12h | No renal dose adjustment |
There are other more effective, less toxic alternatives for most multidrug-resistant Gram-negative infections. If a polymyxin is required, polymyxin B is preferred for intravenous treatment of systemic infections in adults. For treatment of urinary tract infections, or use via inhalation, use colistin (polymyxin E).
Restricted to ID or Antimicrobial Stewardship
Posaconazole
| Indication | Dosing | Notes |
|---|---|---|
| All Indications (IV or Delayed-release tablet) | 300 mg IV/PO q12h x 2 doses, then 300 mg IV/PO q24h | No renal dose adjustment (avoid IV if possible in patients with CrCl <50 mL/min due to accumulation of IV vehicle) |
Take with food. Posaconazole SOLUTION has more frequent dosing, low bioavailability, and significant food restrictions. Do not substitute SOLUTION for TABLETS or IV without discussion with ID Pharmacy.
Review medications for potential drug interactions.
Posaconazole trough levels should be obtained in most patients, whether receiving the agent for prophylaxis or treatment of fungal infections. Trough samples should be obtained 5-7 days after:
-start of therapy
-change in dose
-change in route of administration
-change in potentially interacting drugs
See UCSF Lab Posaconazole Recommendations for specifics of monitoring.
IV formulation - Restriced to ID or Antimicrobial Stewardship
PO formulation - Restricted to ID or Antimicrobial Stewardship except:
1) Prophylaxis against fungal infections in patients on the hematology/BMT or lung transplant services with an intolerance of or contraindications to voriconazole
2) Suspected or documented fungal pneumonia in the hematology/BMT or lung transplant services
3) Empiric therapy for prolonged febrile neutropenia in hematology/oncology/BMT patient
Pyrazinamide
| Indication | CrCl > 30 mL/min | < 30 mL/min |
|---|---|---|
| All Indications |
20-25 mg/kg PO q24h: 36-39 kg: 750 mg PO q24h 40-50 kg: 1000mg PO q24h 51-59 kg: 1250 mg PO q24h 60-70 kg: 1500mg PO q24h 71-80 kg: 1750 mg PO q24h > 81 kg: 2000 mg PO q24h |
25-30 mg/kg PO three times weekly |
Supplied as 500 mg tablets
This dosing aligns with current practices at the SFDPH TB Clinic (as of 5/2023). For patients in other counties, contact the respective TB clinics for dosing recommendations.
Remdesivir
| Indication | CrCl >30 mL/min | CrCl <30 mL/min |
|---|---|---|
| All Indications | 200 mg IV x1 then 100 mg IV q24h | No adjustment required; vehicle may accumulate in renal dysfunction - consider risk/benefit |
Remdesivir
| CrCl>30 ml/min | CrCl<30 ml/min |
|---|---|
| 200 mg IV x1 then 100 mg IV q24h | No dosage required; consider risk/benefit |
Ribavirin
| Indication | CrCl ≥50 mL/min | 30-49 mL/min | 10-29 mL/min | <10 mL/min |
|---|---|---|---|---|
|
Respiratory Syncytial Virus (RSV) Use total body weight for dosing |
40-49 kg = 400 mg PO q12h 50-59 kg = 400 mg PO qAM PLUS 600 mg PO qPM 60-69 kg = 600 mg PO q12h 70-79 kg = 600 mg PO qAM PLUS 800 mg PO qPM 80-89 kg = 800 mg PO q12h 90 kg or greater = 600 mg PO q8h |
40-59 kg = 200 mg PO q12h 60 kg or greater = 200 mg PO q8h |
200 mg PO daily | 200 mg PO daily (consider contacting ID/ASP pharmacist) |
| Hepatitis C | Dosing is highly individualized. Refer to www.hcvguidelines.org for detailed information on appropriate indications for ribavirin in the treatment of hepatitis C infection. | |||
For treatment of respiratory syncytial virus (RSV) in adult patients at UCSF, refer to the RSV Treatment Guideline.
Rifampin
| Indication | Dosing | Notes |
|---|---|---|
| Mycobacterial infections | 600 mg IV/PO q24h |
No renal dose adjustment |
| Prosthetic device infections | 300 mg IV/PO q12h | |
| Endocarditis | 300 mg IV/PO q8h |
Review medications for potential drug interactions.
Tedizolid
| Indication | Dosing | Notes |
|---|---|---|
| All Indications | 200 mg IV/PO q24h | No adjustment for renal dysfunction |
Restricted to ID or Antimicrobial Stewardship
Tigecycline
| Indication | Dosing | Notes |
|---|---|---|
| All Indications | 100 mg IV x1, then 50 mg IV q12h |
No adjustment for renal dysfunction Severe hepatic dysfunction: 100 mg IV x1, then 25 mg IV q12h |
*There may be certain situations that may require higher dosing (200 mg IV x 1, then 100 mg IV q 12h). Speak to ASP/ID pharmacy if needed.
Restricted to ID or Antimicrobial Stewardship
TMP/SMX (trimethoprim/sulfamethoxazole)
| Indication | CrCl > 30 mL/min | 15-30 mL/min | < 15 mL/min |
|---|---|---|---|
| Systemic GNR infections, Nocardia, Stenotrophomonas maltophilia | 10 mg TMP/kg/day divided Q6-12h | 5 mg TMP/kg/day divided Q6-12h | 2.5 mg TMP/kg Q24h |
| Pneumocystis pneumonia, CNS infections | 15 mg TMP/kg/day divided Q6 – 12h | 7.5 mg TMP/kg/day divided Q12-24h | 5 mg TMP/kg Q24h |
Single-strength (SS) tablet contains 80 mg trimethoprim (TMP)
Double-strength (DS) tablet contains 160 mg trimethoprim (TMP)
*May consider Total BW for serious infections
Tobramycin
| Indication | CrCl > 60 mL/min | 40-60 mL/min | 20-40 mL/min | <20 mL/min |
|---|---|---|---|---|
| High-dose extended interval ("once-daily"): patients with normal renal function who are not morbidly obese or fluid overloaded. | 7 mg/kg IV q24h | Use traditional dosing or contact pharmacy for assistance | ||
| Traditional dosing: patients who do not qualify for high-dose extended interval dosing | 1.6 mg/kg IV q8h | 1.5 mg/kg IV q12h | 1.5 mg/kg IV q12-24h | 2 mg/kg loading dose IV x1, contact pharmacy for maintenance |
Monitoring:
|
Indication |
Monitoring |
|---|---|
| Gram-negative high-dose extended interval ("once-daily") |
Single level: Check random drug level 6-14 hours after the start of infusion. Compare to nomogram. Paired levels: Check peak drug level 1 hour after end of infusion and random level 6-14 hours after infusion. Consult ID pharmacy for assistance. |
| Gram-negative traditional dosing | Paired levels: Check peak drug level 30 minutes after end of infusion (goal 5 - 8 mg/L) and trough level immediately before next dose (goal <2 mg/L). |
Hartford Nomogram (7 mg/kg) for Tobramycin & Gentamicin
How to use Hartford nomogram:
- Using the time of serum level (between 6 and 14 hours after dose) and the resultant serum concentration (mcg/mL), locate the intersecting point on the nomogram. Wherever this point lies is the recommended dosing interval for the aminoglycoside.
- If the serum level falls on the line between intervals, choose the longer interval to ensure an adequate drug-free period after each dose.
- If the serum level is above the q48h interval, convert the patient to conventional dosing.
Valganciclovir (Valcyte)
| Indication | CrCl > 60 mL/min | 40 - 59 mL/min | 25 - 39 mL/min | 10 - 24 mL/min |
|---|---|---|---|---|
| CMV Treatment | 900 mg PO q12h | 450 mg PO q12h | 450 mg PO q24h | 450 mg PO every other day |
| CMV Prophylaxis | 900 mg PO q24h | 450 mg PO q24h | 450 mg PO q48h | 450 mg PO twice weekly |
*IV ganciclovir prefferred for initiation of therapy for unstable renal function or in dialysis
Take with food
Vancomycin IV
Refer to UCSF Adult Vancomycin Interim Guidance. ZSFG: Click here for initial dosing guidance.
Use Vancomycin Dosing Calculator (Excel file) for more precise dose calculation and level-based adjustment.
Vancomycin PO
| Indication | Dose | Notes |
|---|---|---|
| Clostridioides difficile infection: initial episode, non-fulminant | 125 mg PO QID | No renal dose adjustment |
| Clostridioides difficile infection: fulminant | 500 mg PO QID* |
*Consider additional rectal instillation
See IDMP guidelines for greater detail and vancomycin taper dosing: https://idmp.ucsf.edu/content/management-clostridium-difficile-infection-adults
PO vancomycin is NOT sufficiently absorbed to treat systemic infections
Voriconazole
| Indication | Dosing | Notes |
|---|---|---|
| All Indications, IV Route | 6mg/kg IV Q12h x 2 doses, then 4mg/kg IV Q12hr | Risk/benefit consideration for IV formulation for CrCl<50 mL/min, as IV vehicle accumlates; consider PO |
|
All Indications, PO Route In obese patients consider a weight-based PO regimen (4mg/kg q12H based on adjusted body weight), Consult ID or ASP for assistance. |
400mg PO Q12h x 2 doses, then 200mg PO Q12* | No renal dose adjustment |
Review medications for potential drug interactions.
Voriconazole has high inter- and intra-patient variability. Voriconazole trough levels should be obtained in most patients, whether receiving the agent for prophylaxis or treatment of fungal infections. Trough samples should be obtained 3-5 days after:
-start of therapy
-change in dose
-change in route of administration
-change in potentially interacting drugs
See UCSF Lab Voriconazole Recommendations for specifics of monitoring.
Note: The manufacturer recommends avoiding IV voriconazole in patients with renal impairment due to potential accumulation of the excipient sulfobutylether-beta-cyclodextrin, which may lead to kidney injury. However, limited data suggest that patients with baseline kidney impairment may safely receive short durations of IV voriconazole (Kim 2016; Lilly 2013; Neofytos 2012; Oude Lashof 2012).
Restricted to ID or Antimicrobial Stewardship except:
1) Prophylaxis against fungal infections on the hematology/BMT/lung transplant services
2) Suspected or documented fungal pneumonia in the hematology/BMT/lung transplant services
3) Empiric therapy for prolonged febrile neutropenia in hematology/oncology/BMT patient
